INDICATIONS FOR IMMUNOTHERAPY
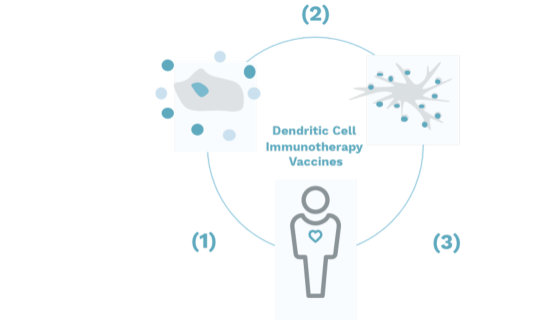
An effective way to combat tumor cells remaining after radical treatment and creating a threat of relapse of the disease is to activate the patient's own immune system. Modern methods of personalized cell-mediated immunotherapy are using the patient’s own immune cells programmed to attack and destroy cancer cells. The main task of activating the antitumor immunity of a patient is performed by his own dendritic cells (DC), which are the specialized and most powerful antigen-presenting cells, and with the help of which one can initiate a directed immune response against the tumor.
Immunotherapy is indicated for patients with chemoresistant solid tumors (cancer of the stomach, pancreas, breast, bladder, lungs, etc.) Usually - after a radical surgery and standard postoperative treatment (chemoradiotherapy).
SAFETY & BENEFITS
The course introduction of an antitumor vaccine allows stabilization of the oncological process and the timing of subsequent lines of chemotherapy, and also often increases the disease-free period, and, in general, life expectancy. The clinical effect of one course of immunotherapy (5 doses) is manifested by stabilization of the tumor process (in 40 - 50% of cases), sometimes with partial or full regression (5 - 15%), and can last, on average, from 3 months to a year or more .
The maximum clinical effect of DC can be expected if it is carried out continuously in the adjuvant mode after removal of the primary lesion (after surgery) in order to prevent tumor recurrence and to destroy invisible micrometastases.
Clinical studies on the use of DCs for the treatment of malignant neoplasms have been conducted in leading medical centers in the world for more than 20 years, and the results of most of them are encouraging - the safety of using DCs, activation of the immune system, and significant clinical effect have been proved.
There is a likelihood of low efficiency of DCs when using standardized synthetic tumor-associated antigens for their production, the expression of which by the tumor is not confirmed by immunohistochemical data. That is why an immunohistochemical analysis is highly desirable.
It is proved that the use of autologous DCs is completely safe, does not cause allergic, toxic reactions, does not suppress immunity and does not stimulate tumor growth. Direct administration of the vaccine can be accompanied by a short-term (1-2 days) increase in body temperature up to 38 ° C, redness and swelling of the injection site, which do not require medical attention and pass on their own.
TREATMENT REGIMEN
Stage 1 - COMPREHENSIVE CHECK-UP
To develop treatment tactics and determine the vaccination schedule, a specialist oncologist of the Department of Regenerative Medicine and Cell Therapy carries out all the diagnosis procedures andcompulsory examinations.
Stage 2 - BIOMATERIAL SAMPLING
The biomaterial for obtaining CELL PRODUCT based on Dendritic Cells is peripheral venous blood (50-100 ml). The biomaterial extraction is a routine medical manipulation. From a single volume of blood, 1 dose of the vaccine is produced. The number of dendritic cells is determined by the number of native monocytes in the patient’s biomaterial - with their low content in the blood, the number of DCs will also be low.
Stage 3 - BIOMATERIAL PRODUCTION
Monocytes isolated from blood are “loaded” with tumor antigens in the laboratory, which allows to obtain mature dendritic cells that can activate the immune response. Individual antigenic tumor antigens (tumor lysates), as well as synthetic standardized tumor-associated antigens, are used as the antigenic material for creating the vaccine.
Stage 4 - BIOMATERIAL TRANSPLANTATION
An anti-tumor vaccine based on DC is administered by a course of 5 therapeutic doses. The administration of the vaccine (1-2 ml) is a routine medical manipulation, performed subcutaneously in the shoulder or forearm, is performed on an outpatient basis in a treatment room.
Immediately after vaccination, the patient is observed for 1 to 2 hours in a ward.